Modern pathology labs have moved far beyond simple sample testing and now rely on speed, accuracy, and compliance to define success. As the volume of diagnostics increases and regulation becomes stricter, traditional paper-based workflows are being replaced by smart automation. That’s why we’re observing a growing popularity of LIS software for pathology labs.
It streamlines everything from tracking specimens and managing tests to reporting results and controlling quality. For healthcare companies and pathology chains, investing in LIS is not just about improving operations. It’s a strategic step to prepare for the future.
Over the years, we’ve seen how the smallest design choices in LIS architecture ripple into efficiency and long-term scalability. IdeaUsher has been in those trenches, building LIS platforms where a single integration decision saved months of operational headaches. Through this blog, we aim to pass along those lessons and discuss the costs of building LIS software for pathology labs, helping you approach your platform development with a clear understanding.
Key Market Takeaways for LIS Software
According to MordorIntelligence, the global market for pathology-focused laboratory information systems is growing quickly, moving from USD 3.19 billion in 2025 to a projected USD 7.19 billion by 2030 at a CAGR of 12.79%. This sharp rise reflects the need for better sample management, automation, and regulatory compliance, as pathology labs modernize to keep pace with rising diagnostic demands.
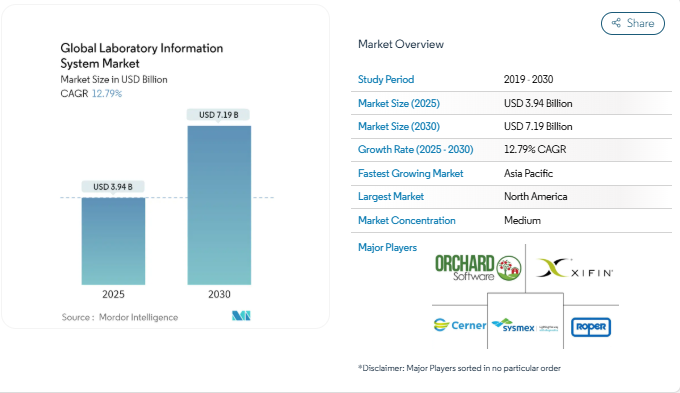
Source: MordorIntelligence
LIS platforms are becoming essential to pathology lab operations because they improve workflows, support complex testing, and connect easily with electronic health records. Cloud access, interoperability, and data analysis give labs the flexibility to manage large amounts of data while keeping up with the demand for personalized medicine and quicker diagnostics.
Mergers and acquisitions are influencing the competitive landscape. Oracle’s purchase of Cerner in 2021 added strong cloud and data management features to Cerner’s LIS portfolio.
Meanwhile, Clinisys’s acquisition of Orchard Software in 2026 created the largest global LIS provider, with over 4,000 labs in 39 countries. These changes expand digital pathology capabilities and enhance long-term support for labs around the world.

Understanding an LIS Software for Pathology Labs
A laboratory information system is a tailored software solution that simplifies and manages the entire diagnostic testing process. From the moment a test is ordered to when the final report is delivered, it serves as the central system for handling patient data.
It automates key tasks like test ordering, specimen tracking, quality control, and interfacing with lab instruments. The LIS also ensures accurate diagnostic reports are generated and seamlessly shared with clinicians and integrated into Electronic Health Records.
The Difference Between LIS vs. LIMS
The key difference is that an LIS is built around the patient while a LIMS is built around the sample. An LIS follows every detail of a patient’s case to make sure the right diagnosis reaches the right person. A LIMS on the other hand is designed for batch testing in research or industry where the individual story does not matter.
| Aspect | LIS (Laboratory Information System) | LIMS (Laboratory Information Management System) |
| Focus | Patient-centric | Sample-centric |
| Purpose | Designed for clinical care and patient diagnosis | Built for research, pharma, and industrial labs |
| Tracking | Organizes blocks, slides, stains, and results for each patient case | Manages large batches of samples and aggregate data |
| Example Use | A breast biopsy tracked through all derivatives to ensure correct diagnosis | Testing thousands of vials of a drug for purity |
| Outcome | Accurate, timely, and defensible patient reports | Efficient batch processing without patient-level detail |
Core Features of a Modern Pathology LIS
A state-of-the-art LIS goes beyond simple specimen tracking. It is a workflow engine, a compliance tool, and a digital pathology hub. Here are the essential features:
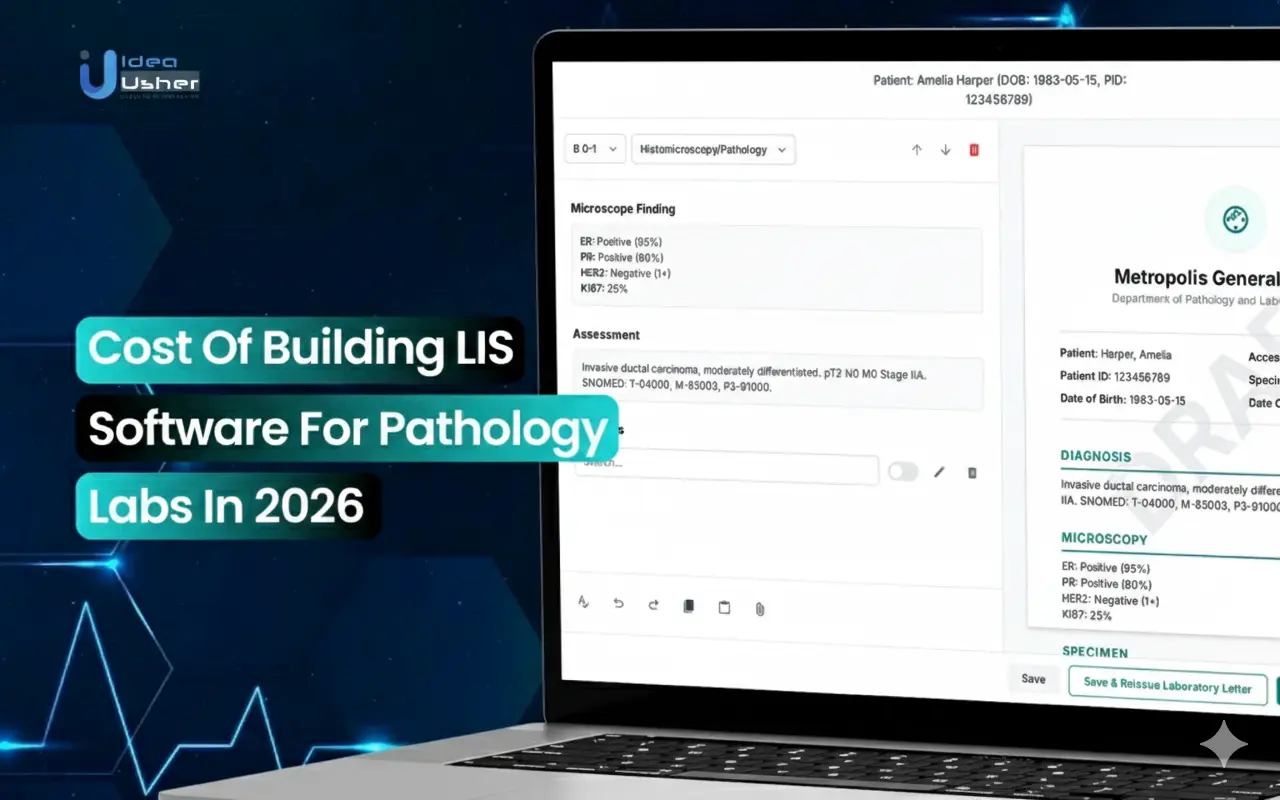
1. Advanced Specimen Tracking and Integrity
An LIS assigns a unique barcode to every specimen so nothing gets lost along the way. It tracks who handled it and when, which removes uncertainty. This level of detail keeps patients safe from mix-ups and makes sure reports stay accurate.
2. Digital Pathology Integration
A modern LIS links straight to slide scanners so digital images move into the system with no extra work. By using HL7 and DICOM it keeps every image tied securely to the right patient. This lets pathologists review cases from anywhere and work together with ease.
3. Structured and Synoptic Reporting
An LIS comes with CAP templates that guide pathologists to report cancer findings in a consistent way. It can also turn free text into coded data using SNOMED CT so nothing gets lost in translation. This makes reports useful not just for care but also for research billing and registries.
4. Deep EHR/EMR Interoperability
An LIS takes orders straight from the EHR so staff do not waste time typing them in and mistakes are avoided. Once the report is ready it flows back into the patient record without delay. This means the doctor can see results right away and act quickly for the patient.
5. Intelligent Workflow Automation
An LIS uses smart rules to handle tasks automatically so cases reach the right pathologist and extra tests are ordered when needed. It can even flag urgent results and alert the surgeon without delay. This kind of automation speeds up the process and keeps errors to a minimum.

Why Pathology Labs Are Investing in LIS Software?
For years, labs saw the LIS as a filing cabinet for results, but now it has become the heartbeat of survival and growth. With margins shrinking and diagnostics growing more complex, modern platforms are no longer a back-office tool but the engine of accuracy and efficiency.
This is why labs everywhere are racing to invest and transform their LIS into a true competitive advantage.
1. Operational Pressure and Financial Health
Labs today face relentless demands to improve efficiency while keeping costs in check. Legacy LIS platforms, weighed down by manual workflows, drain both time and revenue. Modern solutions like Epic Beaker and Orchard Harvest change the equation with advanced automation that minimizes waste and maximizes throughput.
The Bottom-Line Impact of a Modern LIS
- Faster Turnaround Times: Tools such as Cerner Millennium use auto-verification to release normal results instantly, while Sunquest Information Systems streamline case movement with intelligent workflow routing.
- Error Reduction: Platforms like NovoPath leverage barcoding and specimen tracking, ensuring every block, slide, and report is tied to the correct patient.
- Staff Efficiency: Systems such as CoPath Plus automate CPT code assignment, freeing technologists to focus on complex cases while accelerating the revenue cycle.
2. The Diagnostic Revolution
The microscope is no longer the sole anchor of diagnostics, the monitor is stepping in. Legacy systems can’t handle the massive digital files that modern practice demands.
The Modern LIS as the Central Hub
Solutions like Proscia Concentriq and Xifin LIS are purpose-built to support whole slide imaging, enabling pathologists to view, annotate, and finalize cases entirely within the LIS. Their open APIs connect seamlessly with AI leaders such as Paige and Ibex, adding advanced detection and grading support directly into daily workflows.
3. Deeper Integration and Interoperability
Labs don’t operate in silos. Their value depends on how well they connect with the broader healthcare ecosystem.
Beyond HL7 v2 to Modern APIs
- While HL7 v2 remains common, forward-looking platforms are moving ahead. Epic Beaker and Cerner Millennium deliver built-in interoperability as part of larger EHR environments.
- At the same time, systems like Clinisys LIS (formerly Sunquest) and Orchard Harvest are adopting HL7 FHIR APIs, enabling faster, more flexible data exchange across EMRs and partner applications.
4. Unlocking the Power of Data
A legacy LIS locks valuable information away, while a modern system turns data into a competitive advantage.
From Reactive to Proactive with Business Intelligence
- Platforms such as Xifin LIS offer powerful analytics dashboards that track revenue cycle metrics and turnaround times in real time.
- Similarly, Clinisys LIS provides embedded BI tools for deep dives into test volume, reagent utilization, and physician ordering habits. The result is actionable intelligence that directly guides business and clinical decisions.
5. Staying Ahead on Compliance
Compliance failures are costly and damaging. Manual processes make it nearly impossible to keep pace with evolving standards.
Compliance by Design
Modern platforms like NovoPath and CoPath Plus build compliance into the workflow. Synoptic reporting templates enforce CAP cancer protocols, while detailed audit trails safeguard against errors and meet CLIA and HIPAA requirements.
Cost of Building LIS Software for Pathology Labs
Building a custom LIS for pathology labs is a significant investment, but the right approach can make it cost-effective and sustainable. We focus on smart planning and efficient execution so our clients get a powerful system without unnecessary spending.
1. Discovery & Planning (Blueprint Stage)
This stage defines scope and requirements, helping avoid costly rework later.
| Sub-Step | Deliverables | Cost Range (USD) | % of Total |
| Business Analysis & Requirements | Detailed requirements, compliance plan (HIPAA, CLIA, CAP). | $15,000 – $40,000 | 5% – 10% |
| Architecture & UI/UX Design | System design, database schema, wireframes, and prototypes. | $20,000 – $60,000 | 5% – 10% |
| Total Planning | $35,000 – $100,000+ | 10% – 20% |
2. Core Development & Integration (Build Stage)
The heaviest lift in terms of time and cost, this is where coding, integrations, and custom features are built.
| Sub-Step | Deliverables | Cost Range (USD) | % of Total |
| Core LIS Modules (MVP) | Accessioning, sample tracking, results entry, user management. | $50,000 – $150,000 | 15% – 30% |
| Instrument Interfaces | Bi-directional connections to analyzers. | $30,000 – $100,000+ | 10% – 20% |
| EHR/EMR & Billing Integration | HL7 connections to Epic, Cerner, or billing systems. | $25,000 – $75,000+ per integration | 10% – 15% |
| Pathology-Specific Features | Digital pathology, synoptic reporting, automation rules. | $30,000 – $120,000+ | 10% – 20% |
| Data Migration | Legacy or paper records into new LIS database. | $10,000 – $40,000 | 5% – 10% |
| Total Development | $145,000 – $485,000+ | 45% – 70% |
3. Testing, Deployment & Training (Go-Live)
Before launch, the system must be validated, secured, and staff trained.
| Sub-Step | Deliverables | Cost Range (USD) | % of Total |
| QA & Testing | Functional, security, and performance testing. | $20,000 – $70,000 | 5% – 10% |
| Compliance & Validation | Regulatory approvals (CLIA, CAP). | $10,000 – $50,000 | 5% – 10% |
| Deployment Support | Production launch, on-site or remote transition help. | $10,000 – $40,000 | 5% – 10% |
| Training & Documentation | Manuals and role-based training for staff. | $5,000 – $25,000 | 5% – 10% |
| Total Deployment | $45,000 – $185,000+ | 15% – 30% |
4. Post-Launch & Ongoing Costs
Long-term costs often exceed initial build costs over the software’s lifecycle.
| Sub-Step | Description | Cost Range (USD) | Frequency |
| Maintenance & Support | Updates, bug fixes, compliance patches. | 15% – 20% of build cost | Annual |
| Cloud/Hosting | Servers, storage, database. | $500 – $5,000+ per month | Ongoing |
| Licensing | Third-party software or database licenses (if any). | Varies | Annual |
| Feature Upgrades | Enhancements beyond initial scope. | Variable | As Needed |
5. Cost Summary
| Complexity | Cost Range (USD) | Development Time |
| Basic LIS (Small Lab / MVP) | $100,000 – $250,000 | 6 – 9 months |
| Medium LIS (Regional / Multi-Specialty) | $250,000 – $500,000 | 9 – 15 months |
| Enterprise LIS (Full Integrations, Digital Pathology) | $500,000 – $800,000+ | 12 – 18+ months |
These figures are only estimates to give you a sense of the investment involved. The total cost of building a custom LIS can range from $100,000 to $800,000+ USD, depending on scope and complexity. For a more precise quote, you are welcome to reach out to us for a free consultation.

Variable Cost Factors of an LIS Software for Pathology Labs
When you think about the cost of building an LIS, it is rarely the software alone that drives it up. Hidden factors like integrations, compliance timelines, adoption, and data migration can quickly spiral. The real difference comes from tackling these challenges with clarity and smarter planning so the investment actually delivers value.
1. The Integration Labyrinth
Most labs are told that connecting their new LIS is a one-time budget line, but the reality is a spiraling cost center. Every instrument, analyzer, EHR (Epic, Cerner), or digital scanner demands a custom interface project.
Even a “simple” connection can cost $5,000, while a full-featured EHR interface runs between $20,000 and $50,000. With 15+ instruments, a $75,000 line item can snowball into a $300,000+ expense.
Our Solution – API-First & Middleware Mindset
Instead of relying on bespoke integrations, we use a middleware layer with standardized protocols (HL7 FHIR, DICOM) that serves as a universal translator.
The Cost Transformation: This model cuts new interface costs by 40–60%. A $20,000 EHR integration is reduced to an $8,000–$12,000 configuration. What was once unpredictable and bloated becomes a manageable, repeatable investment.
2. The Compliance Maze
Too often, compliance is left until the end, and that is when the bills start piling up. Labs spend anywhere from $50,000 to $150,000 on outside help just to patch gaps and chase errors. It turns into a constant tax that eats away at confidence and budgets.
Our Solution – Compliance by Design
We bake compliance into both code and process. Using a validated agile framework and compliance-first architecture, we remove the need for last-minute remediation.
The Cost Transformation: Instead of open-ended consulting bills, validation becomes a fixed-fee engagement ($20,000–$40,000). This shift replaces surprise costs with predictability, turning compliance from a liability into a built-in safeguard.
3. The Timeline Trap
Traditional LIS projects drag on for 12–18 months. During that time, labs shoulder $150,000–$400,000 in team costs before they process a single test, while competitors move ahead.
Our Solution – Phased Minimum Viable LIS Launch
We don’t wait for a “big bang.” Instead, we partner with labs to define and deliver a working, Minimum Viable LIS in just 3–4 months.
The Cost Transformation: An $80,000–$150,000 phased launch means labs can see ROI in the first quarter, not the third year. Costs are spread across value-driven milestones, allowing efficiency gains to fund future development.
4. The Human Factor
When people struggle to use a system, the real cost shows up quietly in lost time and errors. Labs can lose $20,000 to $50,000 every year on extra training and missed efficiency. Adoption is not just a nice-to-have; it directly protects the bottom line.
Our Solution – Immersive Adoption & Intuitive UI/UX
We design adoption as a product feature. Pathologists are co-creators through design sessions, and training is role-based, scenario-driven, and practical.
The Cost Transformation: Our onboarding program is a fixed-cost ($10,000–$25,000) investment that pays for itself immediately. By ensuring users embrace the system from day one, we eliminate recurring efficiency losses and lock in ROI.
5. The Data Migration Abyss
Migrating years of patient data from legacy systems is often underestimated. A “simple” transfer quickly devolves into weeks of cleansing, validation, and manual entry—pushing projects $15,000–$75,000+ over budget.
Our Solution – ETL Pipelines with Rigorous Validation
We use automated Extract, Transform, Load (ETL) pipelines to map, clean, and validate data integrity before it enters the new LIS.
The Cost Transformation: After discovery, we scope migrations as fixed-price projects ($20,000–$50,000, depending on data volume). This replaces uncertainty with precision, eliminating surprise overruns and safeguarding patient record integrity.
Conclusion
Building LIS software requires a significant investment but can yield strong returns. Long-term success relies on focusing on interoperability, compliance, and being ready for digital pathology. With the right strategy, a custom LIS can improve operations and open new revenue streams for healthcare organizations. Idea Usher helps groups design, build, and connect scalable LIS platforms that fulfill current needs while getting ready for future growth.
Looking to Develop a LIS Software for Pathology Labs?
At IdeaUsher, we design custom LIS platforms that turn pathology labs into hubs of efficiency, accuracy, and growth. Our goal is simple, build technology that works with you, not against you.
Why Partner with IdeaUsher?
We bring together healthcare know-how and world-class engineering to deliver solutions that fit your workflows perfectly.
- Elite Technical Power: With more than 500,000 hours of coding experience, our ex-MAANG/FAANG engineers deliver LIS systems that are secure, scalable, and future-ready.
- Pathology Expertise: From structured synoptic reporting to digital pathology integration and complex case tracking, we build features that pathologists actually use and value.
- Seamless Workflows: Barcode-based specimen tracking, mobile PPID apps, and automation tools reduce errors and speed up every stage of the lab process.
What We Deliver
- Unified CP and AP modules for a complete diagnostic view
- Auto-verification engines to cut turnaround times
- Outreach portals to grow new revenue streams
- HIPAA and CLIA-compliant architecture at the core
Ready to Get Started?
Take a look at our latest projects and see how we’ve helped labs like yours achieve transformation and measurable growth.
Work with Ex-MAANG developers to build next-gen apps schedule your consultation now
FAQs
A1: Building a Laboratory Information System usually takes between 9 to 18 months, with the timeline influenced by factors such as the size of the lab, the number of modules needed, system integrations, and regulatory compliance. More complex labs with advanced workflows and extensive interoperability needs will naturally require more development time.
A2: Yes, modern LIS solutions are designed to integrate seamlessly with EHR and EMR systems through HL7 and FHIR APIs, allowing real-time, secure data exchange. This ensures that lab results flow directly into patient records without manual entry, improving efficiency and reducing errors.
A3: An LIS must adhere to strict regulatory standards to ensure data security, accuracy, and patient safety. Key standards include CAP and CLIA for laboratory quality, HIPAA and GDPR for data privacy, and ISO 15189 for international accreditation of medical laboratories.
A4: A custom LIS gives laboratories full control over their workflows, scalability, and future enhancements, without being limited by the constraints of off-the-shelf software. It also helps avoid vendor lock-in, ensuring the system can evolve with the business’s needs and maintain a competitive edge.