The world of clinical research is changing fast in 2025, and a big part of that change is the rise of patient-centric apps. These apps, like Clara Health, are putting patients at the center of the process, making clinical trials easier to access and more inclusive. They simplify things like recruitment, improve communication between patients and researchers, and even allow for trials to be conducted remotely.
It’s not just about technology; it’s about improving the overall experience for patients and making trials more diverse, while also collecting real-time data that can lead to better outcomes. This shift is making clinical trials feel more personal and more in tune with the needs of those involved.
The future of clinical trials is digital, and it’s all about putting the patient first. Apps like Clara Health are leading the way, making it easier for people to participate in trials by offering features like digital consent forms, telemedicine, and automated follow-ups. These tools not only make trials more accessible but also ensure that patients stay engaged throughout the process. Drawing from our years of experience in healthcare innovation, IdeaUsher understands the complexities of building such apps. That’s why we are sharing this blog, to help you develop a solution that empowers patients and improves trial management.
Key Market Takeaways for Clinical Trial Apps
According to ForinsightsConsultancy, the Digital Clinical Trial Platforms market is experiencing robust growth, with an impressive CAGR of 12.3%. The market, valued at $2.2 billion in 2023, is expected to reach $5.5 billion by 2030. This rapid expansion underscores the growing role of digital technologies in transforming clinical research and healthcare.
Source: ForinsightsConsultancy
The surge in healthcare app adoption is a key driver of this growth. By 2025, Americans are projected to spend up to five hours daily on smartphones, integrating health management tasks such as fitness tracking, telemedicine, and chronic disease management. These apps leverage advanced technologies like AI, cloud computing, and wearables, streamlining patient care and enabling data-driven decisions that improve outcomes.
Partnerships between technology providers and healthcare organizations are accelerating innovation in the clinical trial space. Companies like Medable and CVS Health are working together to expand patient access to trials through retail clinics. Meanwhile, platforms like Medidata’s AppConnect and Triall’s blockchain integration with Crucial Data Solutions are enhancing trial efficiency, improving data integrity, and fostering deeper participant engagement.
What Is the Clinical Trial App Clara & How it works?
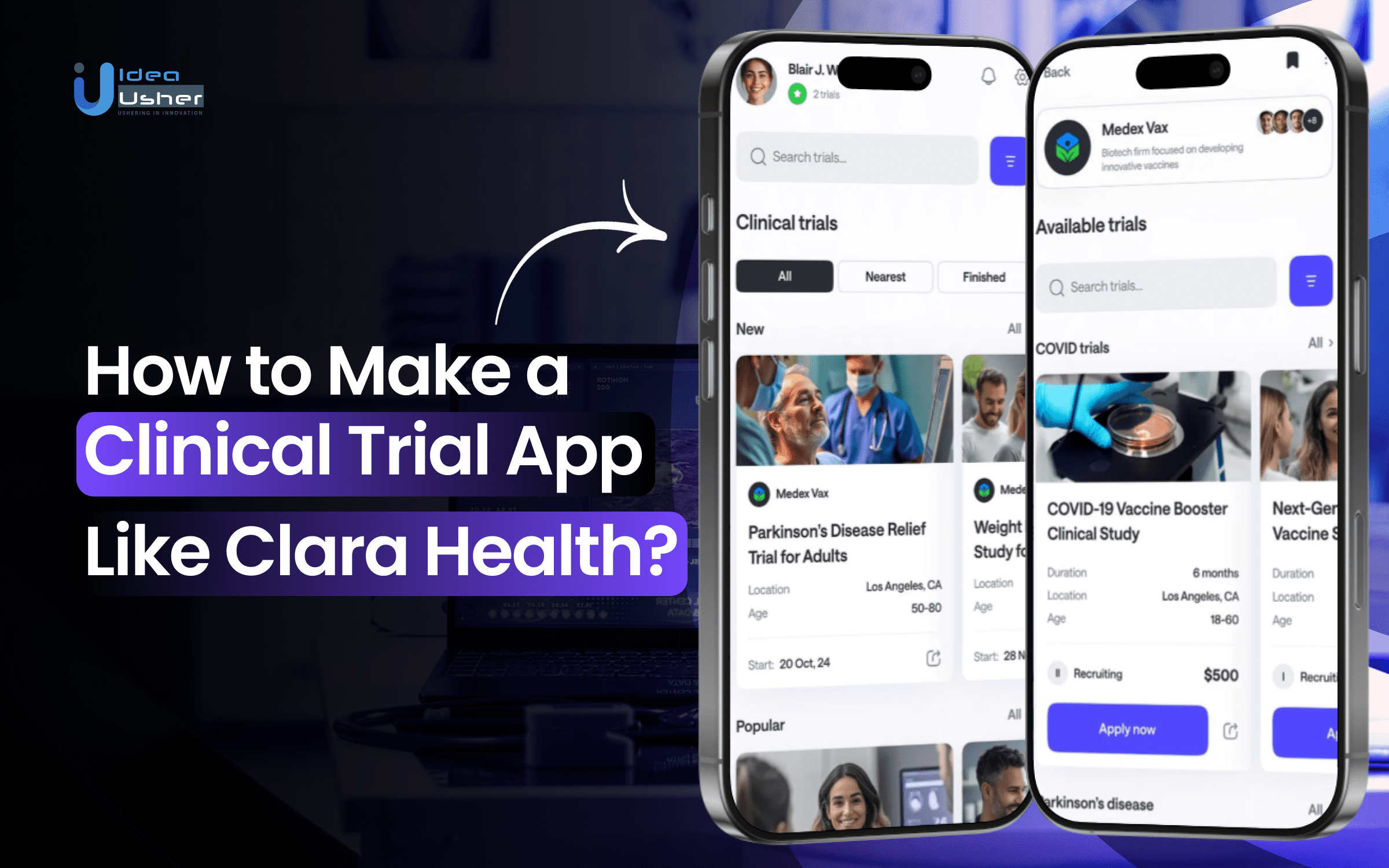
Clara Health is a digital platform designed to connect patients with clinical trials that match their specific needs. By using a blend of advanced technology and personalized human support, Clara Health helps patients find trials suited to their medical background, location, and preferences, improving the efficiency of the recruitment process for both patients and trial organizers.
Who Benefits from These Platforms?
- Pharma and Biotech Companies – They can enroll patients faster and reduce dropout rates, making clinical trials more efficient and less costly.
- Hospitals and Research Sites – Clara Health streamlines the patient recruitment process, reducing the time and effort required to find the right participants.
- Patients – Accessing clinical trials becomes easier, with tailored support and simplified processes throughout their journey.
How Does The App Work?
1. Patient Matching:
Clara Health uses AI-powered technology to match patients to appropriate trials based on their health history, current condition, and specific trial requirements. This helps ensure patients are matched with the most relevant studies, improving their chances of qualifying and participating.
2. Enrollment & Onboarding:
The process of signing up for a clinical trial is simplified through digital consent forms and remote eligibility checks. Patients can complete these steps from home, eliminating the need for in-person visits and speeding up the enrollment process.
3. Patient Engagement & Retention:
Keeping patients engaged throughout the trial is essential for its success. Clara Health helps with this by providing appointment reminders, educational content, and progress tracking tools. These features ensure patients stay informed and motivated to stick with the trial.
4. Data Capture & Compliance:
Clara Health integrates with existing healthcare systems, such as EHRs, to securely capture and track patient data in real-time. This ensures that clinical trials comply with industry regulations while also collecting accurate data through tools like ePRO (electronic patient-reported outcomes).
5. Support System:
One of the key strengths of Clara Health is its 24/7 support system. Whether it’s via chat, phone, or even in-person visits, patients can reach out to care coordinators for assistance at any time, making the process less daunting and more personal.
What Makes Clara Health Stand Out?
AI-Driven Matching & Human Support:
- AI analyzes patient profiles and matches them with suitable trials
- Care coordinators provide ongoing support, guiding patients through each step.
Local Community Recruitment:
- Focuses on recruiting participants from local neighborhoods.
- Ensures diverse, representative trial populations for more reliable results.
Streamlined Trial Management:
- End-to-end digital management simplifies the process from recruitment to data collection.
- Reduces administrative burden, allowing trial sites to focus on research.
Why This Matters for Healthcare?
This matters because faster patient enrollment and retention lead to quicker treatments reaching those who need them. Plus, streamlined processes help trials run smoothly, saving time and resources while improving data quality.
1. Patient-Centricity Drives Business Success:
The faster patients are enrolled in clinical trials, the sooner new drugs and treatments can reach the market. This accelerates revenue for pharma companies and ensures that treatments get to patients who need them quicker. In addition, higher retention rates lead to more reliable trial data, which in turn supports better product development.
2. Decentralized Trials Are on the Rise:
The pandemic highlighted the need for remote monitoring, telehealth consultations, and even home-based sample collection. Clara Health helps to bring these decentralized methods to life, enabling trials to happen without patients needing to visit a site for every visit.
3. Compliance and Data Security:
Clinical trials must adhere to strict regulations, such as FDA 21 CFR Part 11, HIPAA, and GDPR. Clara Health ensures that these standards are met through secure data handling, including the use of technologies like blockchain for audit trails and FHIR (Fast Healthcare Interoperability Resources) for smooth data sharing across systems.
Business Model of the Clara Health App
Clara Health operates a platform designed to make it easier for patients to discover and join clinical trials. The company’s core mission is to accelerate medical research while broadening access to new treatments. Its services guide patients through every step of the process, from identifying suitable trials to enrolling, staying engaged, and adhering to study protocols.
Revenue Streams and Clients
Clara Health earns revenue by working with clinical trial sponsors, including pharmaceutical companies and contract research organizations. These clients pay Clara for a range of services:
- Patient recruitment and enrollment support
- Retention programs to keep participants engaged and compliant
- Guidance on trial protocol design to improve participant experience
- Site selection strategies
- Data-driven engagement plans tailored to target populations
This model directly addresses one of the most persistent challenges in clinical research: recruiting and retaining qualified participants. More than 80% of trials face delays due to recruitment issues, and Clara positions itself as a partner that helps reduce these bottlenecks.
By combining technology, patient advocacy teams, and proprietary data, Clara delivers a patient-first approach that helps sponsors complete studies more efficiently.
Financial Performance and Funding
Clara Health raised about $10.59 million over 10 funding rounds, attracting investment from firms such as Founders Fund and Khosla Ventures. As of early 2018, the company’s valuation was around $10 million. In January 2020, Clara closed a Series A round that brought in $7.09 million to expand operations and enhance its platform.
In March 2022, Clara Health was acquired, although the specific terms of the deal were not publicly shared. While the company spent its early years largely pre-revenue, it has since grown to support a broad spectrum of clinical studies, reflecting a shift toward sustainable commercial operations.
Benefits of Patient-Centric Clinical Trial App For Healthcare Platforms
A patient-centric clinical trial app speeds up recruitment, boosts participant engagement, and makes trials more inclusive. It also streamlines operations, ensuring better data integrity and regulatory compliance for healthcare providers.
1. Accelerated Recruitment and Enrollment
By streamlining the process of identifying and onboarding qualified participants, a patient-centric app helps speed up recruitment for clinical trials. Participants can easily find trials that match their conditions, and the app ensures faster and more efficient enrollment, reducing delays in getting studies underway.
2. Improved Retention and Engagement
The app’s interactive features, such as reminders, educational resources, and personalized support, significantly improve patient engagement. With these tools, participants are more likely to adhere to protocols, attend visits, and stay committed to the trial, reducing dropout rates and enhancing overall trial success.
3. Enhanced Diversity and Inclusion
A mobile app can reach a broader audience, including underserved populations, through improved access to trials in remote or rural areas. This expanded reach helps ensure that clinical trials include diverse patient groups, leading to more representative data and better health outcomes across various demographics.
4. Operational Efficiency for Sponsors and Sites
With reduced manual processes, real-time data collection, and automated workflows, a patient-centric app enhances operational efficiency for sponsors and clinical trial sites. It simplifies tracking and monitoring, minimizing administrative burden and allowing staff to focus on higher-value activities.
5. Regulatory Readiness and Data Integrity
The app ensures that clinical trials maintain a high standard of data integrity with strong audit trails. By complying with international regulatory requirements, it mitigates risks and supports seamless interactions between sponsors, regulatory bodies, and trial sites, ensuring compliance and reducing the likelihood of data errors.
Features to Include in a Clinical Trial App like Clara Health
After developing many clinical trial apps, we’ve figured out which features really hit the mark for users. Through consistent feedback and learning from past iterations, we’ve pinpointed the elements that not only make the app easier to use but also create a more positive and engaging experience for participants. Here’s what we’ve found works best:
Patient Facing Features
1. Intelligent Patient-Driven Scheduling
Users love having control over their schedules. Features like rescheduling requests and automated travel integration save a lot of time and stress. Patients can take charge of their appointments and logistics without needing to make endless calls, making the whole process far more efficient.
2. “My Trial, My Data, My Consent” Dashboard
Transparency around data is a key factor in building trust. This feature gives users control over what data they share, and they can always review or change consent settings. The added transparency with data access logs helps users feel more secure and in control throughout the trial process.
3. Gamified “Contribution & Impact” Tracking
Patients want to feel that their participation matters. By gamifying the process and showing them how their efforts contribute to the trial’s progress, they feel a greater sense of accomplishment. Tracking milestones and seeing their impact motivates users to stay engaged and committed.
4. Biofeedback & Guided Interventions
Integrating real-time biofeedback has been incredibly effective. Whether helping users manage their stress or guiding them through health interventions, it keeps them engaged and compliant with trial protocols. Real-time feedback makes participants feel like they’re supported every step of the way.
5. Dynamic, Context-Aware “Help-on-Demand”
Sometimes, users just need quick, relevant help. With context-aware support, the app can offer guidance right when it’s needed, whether it’s a tutorial on a tricky form or immediate access to emergency protocol instructions. This kind of immediate assistance takes the pressure off and keeps the process running smoothly.
Sponsor Facing Features
1. Real-time Recruitment Dashboard
Sponsors can see recruitment progress in real time with a clear, visual funnel. Predictive enrollment forecasting helps anticipate trends, while diversity analytics and site performance tracking give sponsors the insights needed to make quick, informed decisions.
2. Data Monitoring & RBQM Hub
All trial data is centralized for easy monitoring. Customizable dashboards track key metrics, and AI identifies anomalies in real time. The risk-based monitoring feature allows sponsors to focus on high-risk areas, and remote data review cuts down on time-consuming site visits.
3. Protocol Deviation & Query Management
This feature automatically flags potential protocol deviations and makes managing data queries easy. Sponsors can resolve issues directly from the dashboard, while root cause analysis helps identify recurring problems across sites, making it easier to fix underlying issues.
4. Site Management Portal
From site selection to activation, this portal helps sponsors manage site performance. Interactive workflows guide sites through tasks like IRB submissions, and real-time access to critical documents ensures everything stays organized and compliant.
5. Financial Management Automation
Automated payments and real-time financial dashboards take the stress out of financial management. Sponsors can track spending, automate invoice generation, and ensure timely payments with full transparency and an audit trail, making the financial side of trials easier to manage.
How to Build a Clinical Trial App Like Clara Health?
When we build a clinical trial app for our clients, we focus on creating a solution that simplifies the trial process while ensuring compliance and user-friendliness. Over time, we’ve honed a development process that meets both functional and regulatory needs. Here’s how we approach it:
1. Defining Your Vision and Compliance Strategy
We begin by understanding the client’s vision and identifying their target users, whether they’re patients, study coordinators, or sponsors. We then ensure the app meets all regulatory requirements, like FDA 21 CFR Part 11, HIPAA, GDPR, and EMA guidelines, to ensure full compliance from the start.
2. Designing Patient and Sponsor Journeys
Next, we design tailored journeys for both patients and sponsors. For patients, we focus on ease of use, from pre-screening to consent and reimbursement. For sponsors, we develop workflows to streamline recruitment and data management, making the trial process efficient for all parties involved.
3. Developnig Core Features and Integrations
We develop key features such as AI-powered patient matching, eConsent, and ePRO/eCOA modules for seamless data collection. Integration with telehealth services and wearable devices ensures real-time patient monitoring. For sponsors, we build powerful dashboards and site management tools for better trial oversight.
4. Building a Robust Data Privacy Framework
Data security is essential. We implement strong encryption, access controls, and audit logs to protect patient information. We also ensure compliance with privacy regulations, giving both participants and sponsors confidence in the app’s security.
5. Creating Intuitive UX/UI for All Stakeholders
User experience is key. We design an interface that’s simple and intuitive, ensuring that even users with limited tech experience can navigate the app easily. Accessibility is a priority, making the app usable for everyone involved in the trial.
6. Lanuch
Before launch, we conduct thorough testing to validate functionality and usability. We work with clients to obtain necessary certifications, like ISO and IEC standards, and prepare the app for regulatory submission, ensuring everything is ready for approval.
Overcoming Challenges of Making a Clinical Trial App
After working with numerous clients in the clinical trial space, we’ve seen the common challenges that tend to arise and have developed effective ways to tackle them. Over the years, we’ve refined our approach to make sure we handle these issues head-on, ensuring smooth trials. Here’s how we manage some of the most common challenges:
1. Patient Dropout and Low Engagement
High dropout rates are a frequent issue, often caused by complex trial protocols, lack of motivation, or poor communication with patients. These issues can seriously impact the overall success of a trial
Solutions:
- Behavioral Nudges – Sending timely reminders via SMS, email, or push notifications can help patients stay on track.
- Gamification – Rewarding participants with points, badges, or milestones encourages continued engagement.
- Live Human Support – Offering real-time support through chat or phone, available 24/7, ensures patients feel supported.
- Simplified User Experience – Breaking down complex trial steps into smaller, manageable tasks with visible progress helps patients stay motivated and engaged.
For example, Clara Health reduces patient dropout by assigning dedicated care navigators who check in weekly, offering personalized support to keep participants on track.
2. Data Security and Privacy Risks
Handling sensitive health data requires strict adherence to privacy regulations like HIPAA and GDPR, and any data breach can result in significant legal consequences and loss of trust. A breach can also derail the entire trial process.
Solutions:
- End-to-End Encryption – We ensure that data is encrypted both in transit and at rest, providing a high level of protection.
- Multi-Factor Authentication (MFA) – Adding additional layers of security, like biometrics or one-time passcodes, ensures that only authorized users can access the data.
- Role-Based Access Control (RBAC) – Limiting data visibility to only authorized users minimizes the risk of unauthorized access.
- Blockchain for Audit Trails – Using blockchain technology to track data access creates immutable logs, ensuring full transparency.
Example: Apple HealthKit uses on-device encryption to protect patient data, maintaining privacy while ensuring data security.
3. Complex EHR/EMR Integrations
Many hospitals and clinics still rely on siloed systems, making it difficult to share real-time data between platforms and slowing down trial progress. The lack of seamless integration can lead to delays and data inconsistencies.
Solutions:
- Adopt FHIR APIs – Using FHIR APIs ensures seamless integration across various EHR systems, making data sharing more efficient.
- SMART on FHIR – These pre-built applications allow for easy integration with systems like Epic and Cerner, reducing complexity.
- Partner with Middleware Solutions – Using services like Redox or Particle Health helps connect disparate systems and improves data interoperability.
Essential Tools & APIs for Building a Clinical Trial App
To build a scalable, secure, and compliant clinical trial platform, such as Clara Health, it’s crucial to choose the right technology stack. Here’s a breakdown of the essential tools, APIs, and frameworks categorized by functionality:
1. FHIR & SMART on FHIR APIs
These technologies ensure seamless interoperability with Electronic Health Records (EHR) systems and provide real-time access to patient data.
- FHIR (Fast Healthcare Interoperability Resources): A widely-used standard for exchanging healthcare data.
- SMART on FHIR: Allows apps to run securely within EHR systems like Epic and Cerner, enabling seamless integration.
Key Providers:
- Google Healthcare API
- Microsoft FHIR Server
- Redox Engine (ideal for enterprise-level integrations)
Use Case: Automatically populating patient eligibility forms with data from EHR systems.
2. eConsent Platforms
Digital consent solutions simplify enrollment and ensure compliance with FDA 21 CFR Part 11. Platforms like Medidata eConsent support remote signatures with multimedia explanations, Veeva eConsent integrates with Veeva Vault EDC for seamless compliance, and DocuSign HIPAA-Compliant eSignatures offers secure, legally binding e-signatures with audit trails.
Use Case: Patients can sign consent forms digitally, reducing delays in the trial enrollment process.
3. Telehealth SDKs
Telehealth SDKs facilitate remote patient monitoring and virtual trial visits, crucial for decentralized trials. Twilio Programmable Video offers secure, HIPAA-compliant video consultations, Vonage Video API provides low-latency, globally scalable video services, and Zoom for Healthcare integrates with EHRs for HIPAA-compliant video conferencing.
Use Case: Investigators can conduct virtual consultations with trial participants, making it easier for patients to remain engaged without needing to travel.
4. Wearable & IoT Integrations
Telehealth SDKs enable remote patient monitoring (RPM) and virtual trial visits, essential for decentralized trials. Twilio Programmable Video offers secure, HIPAA-compliant video consultations, Vonage Video API ensures low-latency, globally scalable video services, and Zoom for Healthcare provides HIPAA-compliant video conferencing with EHR integration.
Use Case: Automatically uploading patient vitals (e.g., heart rate, blood pressure) to the trial database for real-time monitoring.
5. HIPAA/GDPR-Compliant Cloud Hosting
Storing sensitive patient data (PHI) requires secure, compliant cloud infrastructure. AWS HealthLake offers FHIR-native cloud analytics and storage, Azure Health Data Services supports DICOM, FHIR, and IoT data with end-to-end security, and Google
Use Case: Secure, compliant storage of trial data with built-in encryption and automatic backup.
6. AI/ML Frameworks
AI and machine learning automate trial processes like patient matching and predictive analytics. TensorFlow/PyTorch are frameworks for building predictive models, AWS Comprehend Medical uses NLP to extract insights from unstructured notes, and IBM Watson Health offers AI tools to enhance patient engagement and optimize trial outcomes.
Use Case: Using AI-driven chatbots to pre-screen patients based on their medical history, improving trial recruitment.
7. Secure Authentication
Robust authentication mechanisms are crucial for protecting sensitive trial data. Okta offers HIPAA-compliant identity management, Auth0 supports SSO, MFA, and biometric logins, while Microsoft Azure Active Directory provides enterprise-grade identity and access management.
Use Case: Role-based access control for investigators, patients, and administrators, ensuring that sensitive data is protected.
Implementation Checklist
| Category | Tool Options | Compliance Ready? |
| EHR Integration | FHIR, SMART on FHIR, Redox | HIPAA/GDPR |
| eConsent | Medidata, Veeva, DocuSign | FDA 21 CFR Part 11 |
| Telehealth | Twilio, Vonage, Zoom | HIPAA |
| Wearables | Apple HealthKit, Google Fit | GDPR |
| Cloud Hosting | AWS HealthLake, Azure | HIPAA |
| AI/ML | TensorFlow, AWS Comprehend | SOC 2 |
| Auth | Okta, Auth0 | HIPAA |
Use Case: Building a Clinical Trial App For Our Client
A global CRO approached IdeaUsher with a challenge: low diversity (under 15% from underserved communities), high dropout rates (40%), and slow recruitment due to manual screening delays of over six months. Their goal was to create a solution that improved diversity, retention, and speed.
The Solution: A Next-Gen Patient Recruitment Platform
To address these challenges, IdeaUsher designed and developed a comprehensive, AI-powered patient recruitment and engagement platform. This platform was built with a focus on patients, leveraging innovative technologies to streamline processes and improve trial outcomes.
AI-Powered Patient Matching
We integrated SMART on FHIR to pull real-time EHR data from Epic and Cerner, enabling faster, more accurate patient matching. TensorFlow-based algorithms streamlined eligibility screening, matching patients 3x quicker than traditional methods.
Real-Time Wearable Data Capture
By connecting Apple HealthKit and Fitbit, we enabled remote monitoring of patient vitals, ensuring continuous data collection. Automated alerts were sent to investigators in case of any adverse events, improving patient safety.
24/7 Live Chat & Human Support
We embedded Twilio-powered live chat and call support, with bilingual coordinators available round the clock. Personalized reminders and nudges, such as “Your next clinic visit is in 2 days!”, helped keep patients engaged and informed.
Hyperlocal Community Outreach
We partnered with local churches, community clinics, and advocacy groups to build trust in underserved areas. Geo-targeted ads were deployed through a NeighborhoodTrials-style approach, focusing on communities with low trial participation rates.
The Outcome: Faster, More Inclusive Trials
Six months after launch, the platform delivered impressive results:
- 300% Faster Recruitment: Reduced screening time from 90 days to 30.
- 85% Retention Rate: Reduced dropouts with AI reminders and human check-ins.
- 40% More Diverse Enrollment: Doubled participation from Black and Latino patients.
Why This Worked?
| Problem | Solution | Tech Used |
| Low diversity | Community health partnerships + geo-targeting | Facebook Ads API, Localytics |
| High dropout | Live chat + AI engagement | Twilio, Dialogflow |
| Slow screening | FHIR + AI matching | SMART on FHIR, TensorFlow |
Conclusion
The future of clinical trials is patient-centric, and platform owners have a unique opportunity to lead in digital health innovation. In today’s world, patient-focused apps are essential—not optional, to accelerate recruitment, improve trial outcomes, and build trust with diverse patient populations. Explore how IdeaUsher can partner with you to design, build, and integrate compliant, tailored clinical trial platforms that meet your specific goals and drive real impact.
Looking to Develop a Clinical Trial App like Clara Health?
At IdeaUsher, we specialize in creating AI-powered clinical trial apps like Clara Health, designed to accelerate enrollment, improve patient retention, and ensure full compliance. We take a collaborative approach, working closely with you to develop a customized solution that aligns with your specific needs and goals, just like Clara Health, making trials faster, more inclusive, and easier to manage.
Why Choose Us?
- 500,000+ Hours of Expertise: Our team of ex-MAANG/FAANG engineers brings deep experience in developing scalable, secure, and compliant health tech solutions.
- End-to-End Development: From AI-driven patient matching to seamless EHR integrations (FHIR, Epic, Cerner) and real-time wearable data capture, we cover it all.
- Proven Success: We’ve helped CROs, pharma leaders, and startups reduce trial timelines by over 50% while significantly improving diversity.
Ready to Transform Your Clinical Trials?
Explore our latest projects to see how we can help streamline your trials and deliver impactful results.
Work with Ex-MAANG developers to build next-gen apps schedule your consultation now
FAQs
A1: A clinical trial app must comply with regulations like FDA 21 CFR Part 11 for electronic records, HIPAA for data security and privacy, and GDPR for protecting personal data of EU citizens. Depending on the location, local data privacy laws might also apply. Additionally, the app must include validated audit trails and secure eConsent features to ensure compliance throughout the trial process.
A2: The development of a clinical trial app like Clara Health typically takes between 6 to 12 months. This timeline accounts for planning, design, development, testing, and validation phases to ensure the app meets regulatory standards and user needs.
A3: Yes, we can integrate your app with existing EHR/EMR systems through FHIR (Fast Healthcare Interoperability Resources), SMART on FHIR, and custom APIs, enabling secure data exchange between your app, hospitals, and research sites, ensuring smooth workflow and real-time access to patient information.
A4: A truly patient-centric clinical trial app focuses on end-to-end support, easy accessibility, multilingual content, and proactive engagement tools. Features like personalized reminders, real-time support, and clear, user-friendly design make the experience seamless, fostering patient trust and active participation throughout the trial.