AI is really starting to make a difference in the life sciences field, especially when it comes to pharmaceutical R&D. It’s not just about speeding up the process; AI is helping to make drug discovery more accurate and clinical trials more efficient. The more specialized these AI tools become, the better they can handle the complexities of the industry. By 2025, platforms like Certara.AI will likely be game-changers in how research and development is done.
For businesses in pharma and biotech, adopting AI isn’t just about keeping up; it’s about getting ahead, saving time and costs, and staying on top of regulatory changes, all while pushing innovation forward.
We’ve helped businesses integrate AI into their life science platforms to improve research timelines and accuracy. These platforms use machine learning and real-time data analytics to simulate clinical trials, analyze patient responses, and recommend personalized treatment options. IdeaUsher has guided numerous organizations through this process, and we’re using this blog to share our expertise, showing you how to develop an advanced AI-powered life science platform like Certara AI!
Key Market Takeaways for AI Life Science Platforms
According to PrecedenceResearch, the global AI in life science analytics market is experiencing rapid growth, with projections indicating a jump from USD 2.22–2.9 billion in 2024 to USD 6.28–16.7 billion by 2034, driven by a compound annual growth rate of 10.9–21.5%. This growth reflects the increasing reliance on AI-powered analytics to speed up drug development, optimize clinical trials, and personalize medicine. As AI continues to reshape the life sciences industry, it plays a crucial role in analyzing vast datasets, predicting patient outcomes, and discovering new therapies.
Source: PrecedenceResearch
AI life science platforms are gaining momentum, enabling faster and more efficient research. Companies like Tempus, Verge Genomics, and FlyPix are leading the charge by integrating genomic, clinical, and imaging data for advanced analytics. These platforms support breakthroughs in precision medicine and disease modeling.
With advancements in machine learning, natural language processing, and generative AI, researchers can now extract valuable insights from unstructured data, making AI an essential tool for pharmaceutical and biotechnology companies.
Strategic partnerships are driving innovation in AI-driven life sciences. Collaborations like IQVIA and NVIDIA’s efforts to develop healthcare-grade AI solutions and Owkin’s partnership with Sanofi highlight the growing importance of AI in clinical trials and drug development.
Understanding AI Life Science Platforms
An AI life science platform is a cutting-edge digital ecosystem designed to integrate AI, ML, and data analytics to advance research, development, and decision-making in the life sciences sector. These platforms unify diverse data sources, such as biological, chemical, and clinical information, to fast-track processes like drug discovery, clinical trial optimization, regulatory compliance, and ultimately improve patient care.
Their scope includes:
- Processing and analyzing multi-modal data, which includes genomics, proteomics, and electronic health records.
- Automating repetitive tasks, such as the generation of regulatory documents.
- Simulating biological processes like pharmacokinetics and toxicity prediction.
- Offering actionable insights to researchers, clinicians, and regulatory bodies for more informed decision-making.
Types of AI Life Science Platforms
AI life science platforms come in different types, each focusing on key areas of drug development. Some specialize in speeding up drug discovery, while others focus on optimizing clinical trials or automating regulatory compliance.
| Platform Type | Purpose | Examples |
| Drug Discovery-Focused Platforms | Accelerate early-stage drug development. | – BenevolentAI: AI for rare disease target discovery.- Atomwise: AI-powered virtual screening. |
| Clinical Trial Optimization Platforms | Improve trial design, patient recruitment, and monitoring. | – Unlearn.AI: AI-driven digital twins for trials.- Saama AI: Enhances trial analytics. |
| Regulatory Compliance & Documentation Platforms | Automate regulatory submissions and ensure compliance. | – Certara’s CoAuthor™: AI for regulatory writing.- Veeva Vault RIM: Regulatory info management. |
| Multi-Functional Integrated Platforms | Combine drug discovery, trials, and regulatory workflows. | – Schrödinger’s Platform: Integrates drug discovery and simulation.- Insilico Pharma.AI: Covers discovery to trials. |
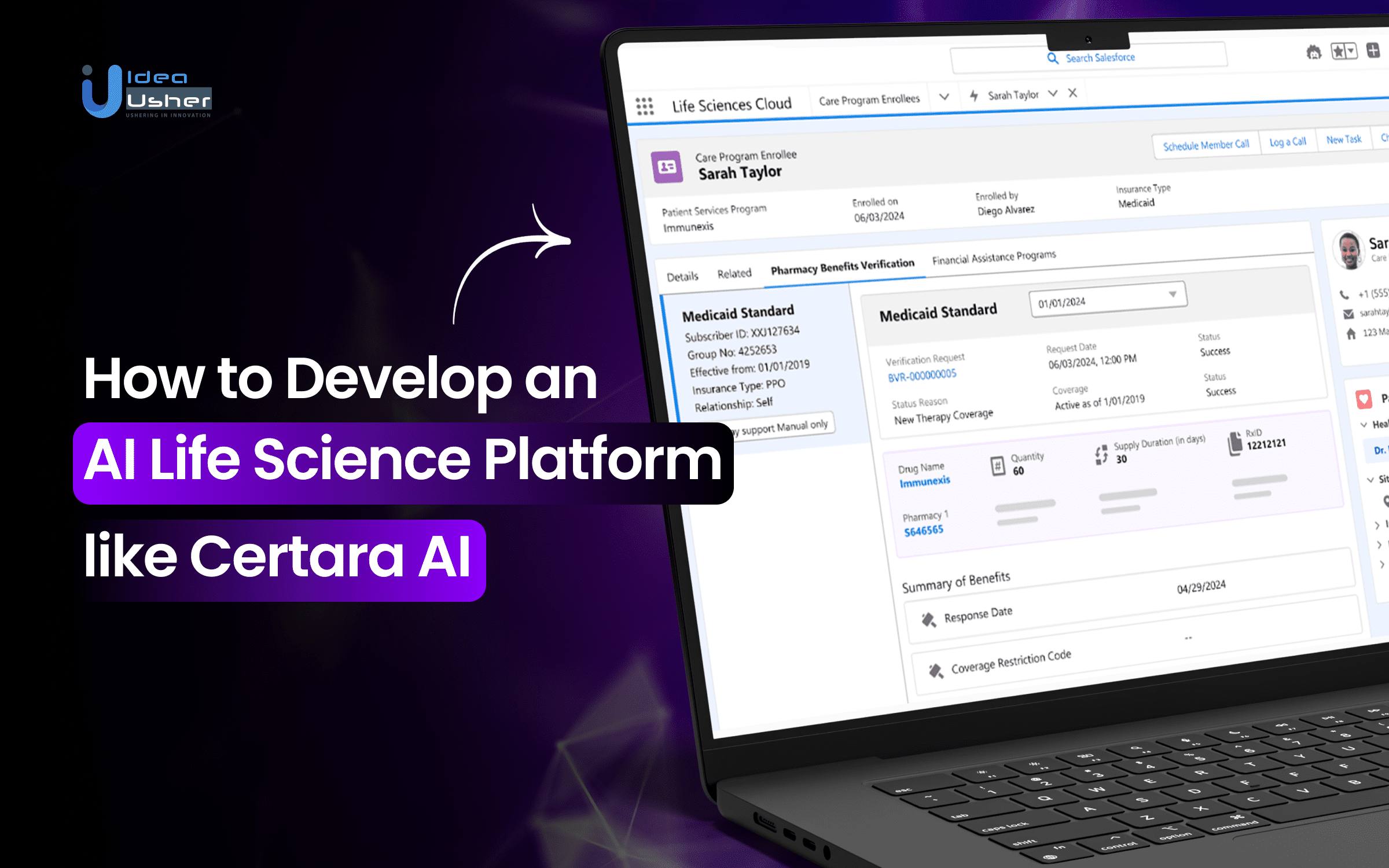
How Does the Certara AI Platform Work?
Certara.AI stands at the intersection of traditional biosimulation and modern artificial intelligence, providing a transformative solution for drug development. By leveraging cutting-edge AI tools and integrating them with tried-and-true biosimulation techniques, Certara.AI helps researchers make more informed, efficient decisions in drug development, from early-stage discovery to clinical trials.
1. Seamless Integration with Biosimulation Tools
At its core, Certara.AI enhances biosimulation capabilities through seamless integration with leading industry tools, ensuring precision at every step of drug development:
- Simcyp Simulator: Direct connectivity allows for physiologically-based pharmacokinetic (PBPK) modeling, ensuring more accurate drug behavior predictions in the human body.
- Phoenix PK/PD Platform: This platform provides population pharmacokinetic analysis, helping to understand drug dynamics across diverse patient populations.
- Automated Data Ingestion: Data from both nonclinical and clinical studies is automatically ingested, streamlining the data analysis process.
2. Enhancing Predictive Modeling with AI
Certara.AI’s ability to enhance predictive modeling sets it apart from traditional biosimulation tools. With its robust AI-driven capabilities, the platform can deliver more accurate and actionable insights:
Machine Learning-Driven Covariate Analysis
AI-driven algorithms automatically identify and analyze key factors (covariates) that affect drug behavior, such as patient age, weight, genetic factors, and more. This leads to a more precise understanding of how a drug will behave in different populations.
Advanced Dose-Exposure-Response Predictions
AI augments traditional pharmacokinetic models to predict how different doses of a drug will impact treatment outcomes, offering a more personalized approach to medicine.
Virtual Patient Population Simulations
Rather than relying on small, homogenous patient groups, Certara.AI simulates virtual populations to predict how a drug will work across various demographics. This helps identify potential issues early in the development process.
Accuracy Improvements
By integrating AI into biosimulation models, Certara.AI improves predictive accuracy by 30-40%, which is crucial for reducing the risks and costs of drug development.
3. No-Code Applications for Drug Development
Certara.AI provides powerful, no-code applications that democratize access to advanced tools, allowing even non-technical users to harness the power of AI in drug development. These applications are designed to streamline various aspects of the process, ensuring faster and more effective outcomes.
CoAuthor™ – Regulatory Writing Simplified
CoAuthor™ simplifies regulatory document creation by automatically populating templates with study data, cutting down on manual work. It ensures version control across drafts and keeps everything aligned with ICH, FDA, and EMA standards, making submissions easier.
D360™ – Empowering Drug Discovery
D360™ streamlines early drug discovery by helping researchers evaluate compounds, analyze their structure-activity relationships, and identify potential drug targets. It also uses predictive ADMET modeling to assess safety and efficacy before clinical trials. Plus, it fosters collaboration, allowing teams to share insights and data more efficiently.
CODEX – Optimizing Clinical Trials
CODEX uses AI to streamline clinical trials by optimizing protocols, forecasting patient recruitment timelines, and predicting site performance. It speeds up protocol development by 25% and boosts enrollment accuracy by 40%, reducing delays and risks. Plus, real-time risk monitoring helps catch potential issues early, keeping trials on track.
4. Security & Compliance Framework
Certara.AI prioritizes enterprise-grade security and data protection, ensuring compliance with the highest standards.
Private GPT Deployment Options: Provides flexibility with air-gapped infrastructure, dedicated instances, or on-premise deployments.
Data Protection Features:
- End-to-End Encryption: Secures data from acquisition to storage.
- Compliance: Certara.AI meets HIPAA/GxP regulations and is SOC 2 Type II certified.
5. Transparent AI Operations
Certara.AI ensures full transparency by tracking all AI-generated content, so everything is reproducible and accountable. It also keeps a clear record of where every data point comes from and monitors model versions, ensuring consistency and reliability. This helps maintain trust and meet regulatory requirements.
Human Oversight Protocols:
- Multi-tier review system ensures scientists, medical experts, and regulatory bodies validate AI-generated results.
- AI Confidence Scoring: Provides transparency about the AI’s certainty in its predictions.
- Alternative Scenario Analysis & Risk-Benefit Visualization: Allows decision-makers to consider multiple scenarios and assess risks versus benefits effectively.
Why Companies Are Adopting AI Life Science Platforms?
Companies are adopting AI life science platforms to speed up drug discovery, improve decision-making accuracy, and ensure regulatory compliance. These tools help cut costs, reduce time-to-market, and minimize trial failures. With AI, businesses stay competitive, attract investment, and position themselves for next-gen innovations.
Faster Drug Discovery
AI accelerates drug discovery by predicting interactions and optimizing compound selection, cutting years off development. During COVID-19, AI helped develop vaccines in under a year, speeding up submission timelines and reducing trial failures.
Improved Decision-Making Accuracy
AI uses data from genomics and real-world evidence to predict treatment responses and detect safety signals early. This leads to personalized dosing, fewer adverse events, and improved trial success rates, saving businesses significantly.
Regulatory Compliance Automation
AI tools like CoAuthor™ automate regulatory document generation and ensure compliance, reducing review cycles and flagging risks early. This streamlines submissions and accelerates approval processes.
Competitive Advantage
AI provides early adopters with a competitive edge, unlocking novel target discoveries and attracting more investment. It helps secure better intellectual property and positions companies for next-gen therapies like gene editing.
Benefits of an AI Life Science Platform for Businesses
Developing an AI life science platform helps businesses scale data handling, adapt models as science evolves, and integrate seamlessly into existing workflows. This leads to significant cost savings, faster R&D cycles, and higher drug development success rates.
Technical Advantages
- Scalable Architecture for Massive Datasets: AI life science platforms process massive amounts of data, using cloud computing and elastic infrastructure to support complex data integration without slowing down.
- Model Flexibility and Adaptability: These platforms adapt easily by continuously improving with new data and fine-tuning models for specific therapeutic areas, like oncology or neurology.
- Seamless Integration with Existing Workflows: Platforms integrate smoothly into existing systems with pre-built connectors and customizable APIs, enabling researchers to access AI insights without changing their workflows.
Business Advantages
- Reduced Operational Costs: AI automation cuts costs, such as a 30-50% reduction in manual data processing and faster regulatory document preparation, helping businesses save millions annually.
- Faster R&D Cycles: AI accelerates R&D stages, like target identification and clinical trials, by automating data analysis, speeding up the timeline to bring new drugs to market.
- Higher Success Rates in Drug Development: AI improves decision-making, reducing Phase II failures and optimizing dosing, turning potential failures into successful, revenue-generating drugs.
How to Develop an AI Life Science Platform like Certara AI?
We specialize in developing cutting-edge AI life science platforms tailored to the unique needs of each client. Whether it’s for accelerating drug discovery, optimizing clinical trials, or streamlining regulatory processes, we follow a structured, client-focused approach to build robust, scalable, and secure AI platforms like Certara AI. Here’s how we develop AI life science platforms for our clients:
1. Define Scope & Use Cases
We begin by understanding your specific goals. Whether you need a platform for drug discovery, clinical trials, or regulatory automation, we work closely with you to define the key use cases and ensure the platform is designed to meet your exact needs.
2. Build Secure & Compliant Data Fabric
Security and compliance are non-negotiable. We build a secure data fabric that incorporates robust data pipelines, end-to-end encryption, and full regulatory compliance. This ensures that sensitive data, whether from clinical trials or patient records, is handled in line with industry standards like HIPAA and GxP.
3. Implement Model-Agnostic Architecture
Our approach is to design a model-agnostic platform, which means you can integrate a wide range of AI models without disrupting the system. This modular design allows your platform to adapt to new AI advancements and provides flexibility as your needs evolve in the fast-paced life sciences industry.
4. Integrate Knowledge Bases
We integrate RAG and proprietary knowledge bases into the platform to maximize its capabilities. By configuring efficient retrieval layers and vector search mechanisms, we ensure the platform can process and extract meaningful insights from vast amounts of specialized scientific data.
5. Add Explainability & Human Oversight
For AI decisions to be trusted, they must be explainable. We incorporate transparency and explainability features in every AI output, ensuring that all insights are auditable and verifiable. With human-in-the-loop functionality, your team can intervene and validate key AI-driven decisions, ensuring they align with real-world goals.
6. Deploy & Scale
Finally, we deploy the platform in the optimal environment, whether on the cloud, on-premise, or hybrid. Our scalable deployment strategies ensure that the platform can grow with your business, supporting increased data loads, user demands, and global usage, while maintaining seamless performance and accessibility.
Key Challenges in Developing an AI Life Science Platform
Over the years, we’ve partnered with various clients to build AI life science platforms, and we’ve learned how to address the challenges that often come up. Here’s how we navigate these obstacles to drive success.
Challenge 1: Data Privacy Concerns
Life sciences handle sensitive data like Protected Health Information (PHI), proprietary compound data, and unpublished clinical trial results. Any breach can lead to regulatory penalties, intellectual property theft, and a loss of patient trust.
The Solution:
- We implement air-gapped infrastructure and strict access controls to protect sensitive data. Using private cloud deployment with dedicated servers, role-based access controls, MFA, and AES-256 encryption ensures that data remains secure both in transit and at rest.
- For maximum protection, we also offer on-premise options. This approach significantly reduces data incidents, as shown by a top pharma company that cut data breaches by 92% while maintaining research pace.
Challenge 2: AI Hallucinations
AI models, especially large language models (LLMs), sometimes generate scientifically inaccurate outputs, such as fictitious citations or incorrect drug indications. This can derail research and lead to regulatory rejections.
The Solution:
- To prevent hallucinations, we use RAG and curated, verified knowledge sources like FDA guidelines and PubMed Central. Additionally, we integrate confidence scoring to flag uncertain outputs, ensuring human experts review critical results.
- Continuous model retraining and scientist feedback loops further reduce inaccuracies. For example, Certara’s CoAuthor™ reduced hallucinations from 15% to under 2% with this approach.
Challenge 3: Integration with Legacy Systems
Many organizations still rely on outdated systems, such as 20-year-old LIMS or proprietary data formats. Integrating these with AI platforms can be costly and time-consuming, threatening the success of the AI project.
The Solution:
- We use an API-first development strategy, providing universal connectors and pre-built adapters for common systems like SAS and Oracle Clinical.
- A middleware layer harmonizes data, and a progressive modernization plan ensures smooth integration with legacy systems, minimizing disruption. One contract research organization cut integration time from 9 months to 6 weeks by using this API-first approach.
Challenge 4: Regulatory Acceptance
Regulators require AI-assisted submissions to have transparent decision-making, reproducible results, and clear accountability. Without these elements, AI submissions are often delayed or rejected.
The Solution:
- We ensure full transparency with audit trails that track model versions, input/output data, and predictions. We also use explainability features like SHAP values and decision pathway visualizations to clarify how AI arrives at its conclusions.
- A governance framework, including AI review boards and mandatory human sign-offs, ensures that every decision is fully accountable and meets regulatory requirements.
Tools & APIs for Building an AI Life Science Platform
Building a powerful AI life science platform requires integrating the right tools, APIs, and frameworks that support data management, AI development, biosimulation, security, and user-friendly interfaces. Below are the key components to help streamline the development of these platforms.
Data Management Layer
1. Enterprise Data Platforms
Databricks Lakehouse
A unified platform that processes structured and unstructured data, with Delta Lake ensuring ACID-compliant management of clinical data. It’s ideal for handling multi-center trial data with built-in HIPAA compliance.
Snowflake Healthcare & Life Sciences Data Cloud
Enables secure data sharing between sponsors and CROs, with native support for FHIR and OMOP data models, plus “Time Travel” for version control and audit compliance.
Neo4j Knowledge Graphs
This tool connects compounds, targets, pathways, and adverse events, helping with drug repurposing analysis. It accelerates relationship queries by 100x compared to traditional relational databases.
AI/ML Development Stack
2. Core Modeling Frameworks
- Hugging Face Transformers: Offers pre-trained models like BioBERT and PubMedGPT, allowing for custom fine-tuning based on specific therapeutic areas.
- TensorFlow/PyTorch: These frameworks support the full machine learning pipeline, from data processing to model deployment. TensorFlow Extended (TFX) helps in building scalable production pipelines, and PyTorch Geometric aids in molecular graph networks.
- NVIDIA Clara: Provides GPU-accelerated biomolecular modeling, enhancing model performance for life sciences applications.
3. Vector Search Infrastructure
- Pinecone: A managed service that allows hybrid search capabilities, useful for FDA guidance document retrieval and scientific literature search.
- Weaviate: An open-source platform with biomedical modules, it automates concept linking, such as connecting “myocardial infarction” to “heart attack.”
- Milvus: Ultra-scalable and perfect for genomic similarity searches, making it highly suitable for precision medicine.
Domain-Specific Scientific Tools
4. Biosimulation Integration
The Simcyp Simulator API offers RESTful endpoints for PBPK modeling, enabling automated virtual population generation through a Python SDK. Meanwhile, Phoenix PK/PD Connectors make it easy to transfer data to and from WinNonlin, integrating seamlessly with the NLME engine for more reliable population modeling
Security & Compliance
5. Certified Infrastructure
AWS HealthLake ensures HIPAA/GxP compliance with de-identification services for real-world data, making regulatory compliance easier. Microsoft Azure Healthcare APIs assist with DICOM image processing and support FHIR servers for seamless data exchange.
Google Cloud Healthcare API enhances patient data analysis and clinical predictions, using BigQuery ML for real-time insights to improve decision-making.
User Experience & Democratization
6. No-Code/Low-Code Interfaces
- Streamlit: Allows for rapid prototyping of biomarker dashboards and interactive visualizations, such as dose-response models, without deep coding knowledge.
- Gradio: A tool that helps share demos for cross-functional teams, including FDA submission document QA checkers.
- Retool: Useful for building internal operational tools, such as clinical trial monitoring portals, to keep teams in sync.
Use Case: AI-Powered Novel Antibiotic Discovery Platform
One of our clients, a mid-sized biotech company, approached us with a pressing challenge: combating antimicrobial resistance. Traditional antibiotic development methods take over a decade and cost upwards of $1 billion. Our task was to accelerate the discovery of new antibiotics using an AI-driven platform, cutting costs and time to market.
Our Solution: AI-Driven Antibiotic Development
We developed an AI-driven platform that accelerated antibiotic discovery by screening novel compounds, validating them through biosimulation, and automating regulatory and patent drafting.
AI-Powered Compound Screening
We utilized generative AI models like REINVENT and MegaSyn to design novel antibiotic molecules. These models were trained on existing antibiotics and bacterial resistance mechanisms, while ADMET prediction models (e.g., DeepTox) filtered out non-viable candidates early in the process.
Knowledge-Enhanced RAG for Scientific Literature
The platform incorporated Retrieval-Augmented Generation (RAG) to retrieve and cross-reference the latest PubMed and MIC studies via Pinecone and Weaviate. This allowed the platform to focus on high-priority pathogens, such as MRSA and CRE, and auto-generate hypotheses about the mechanisms of action using BioBERT.
Biosimulation for Rapid Validation
Molecular dynamics simulations using GROMACS predicted binding affinity, and bacterial growth models like COMBAT-TB simulated the efficacy of candidate molecules. The AI narrowed down the options, identifying the top 3-5 lead candidates for lab testing, dramatically reducing the number of possibilities from over 1,000.
Automated Regulatory & Patent Drafting
The platform leveraged NLP tools similar to CoAuthor™ to automatically generate IND-enabling study reports and patent applications. This streamlined the regulatory submission process, ensuring compliance with FDA’s AMR Action Plan and automating prior art searches.
Outcome: From Discovery to IND in <3 Years
- 50% faster hit-to-lead optimization vs. traditional methods.
- Identified 2 novel antibiotic candidates with activity against pan-resistant Acinetobacter.
- Reduced preclinical costs by 40% by minimizing failed experiments.
- First-ever AI-assisted antibiotic submission under FDA’s AMR Action Plan.
Conclusion
AI life science platforms, such as Certara.AI, are revolutionizing research and development by providing faster, more accurate insights while ensuring regulatory compliance. Businesses that embrace these technologies early will gain a competitive edge in speed and precision, making their processes more efficient. At Idea Usher, we specialize in helping enterprises integrate AI-driven solutions into new or existing platforms, ensuring they unlock the full potential of these innovations for maximum impact.
Looking to Develop an AI Life Science Platform?
At Idea Usher, we partner with biotech firms, pharma leaders, and healthtech innovators to develop cutting-edge AI life science platforms, delivered faster, smarter, and with enterprise-grade precision. We design tailored AI solutions to streamline drug discovery, enhance clinical trial processes, and ensure regulatory compliance.
Why Choose Us?
- 500,000+ Hours of Coding Expertise – Our ex-MAANG/FAANG engineers bring deep expertise in AI, biosimulation, and regulatory-compliant architectures.
- Certara-Level AI Solutions – We create solutions trusted by the industry, from RAG-powered drug discovery to automated regulatory submissions.
- End-to-End Development – From AI models and data pipelines to security and compliance, we handle every aspect of the platform development process.
See Our Work in Action. Let us help you unlock the full potential of AI in life sciences.
Work with Ex-MAANG developers to build next-gen apps schedule your consultation now
FAQs
A1: Building an AI life science platform typically takes between 6 to 12 months, depending on the complexity, compliance needs, and data availability. A clear understanding of your goals and a well-defined scope can help streamline the process and ensure timely delivery.
A2: AI doesn’t replace scientists in drug discovery; instead, it enhances their capabilities. By providing faster insights and automating repetitive tasks, AI allows researchers to focus on high-level decision-making and creative problem-solving, but human expertise is still essential for interpreting results.
A3: Yes, AI can be seamlessly integrated into existing life science platforms. With API-first and modular architectures, AI solutions can be layered onto current systems, enhancing functionality without the need for a complete overhaul, making integration smooth and cost-effective.
A4: To ensure regulatory compliance, you should implement auditable data processes, transparent and explainable AI models, and human-in-the-loop workflows. Aligning your platform with FDA/EMA standards guarantees that it meets the necessary regulatory requirements while maintaining high-quality outcomes.